About the Doyle Lab
Research Interest
The Doyle Lab is dedicated to investigating how the immune system can be modulated to improve stroke recovery. Stroke is a leading cause of disability worldwide, and many survivors experience long-term cognitive and physical impairments. Our research aims to identify how the immune system can be targeted to promote tissue repair and improve functional outcomes after stroke.
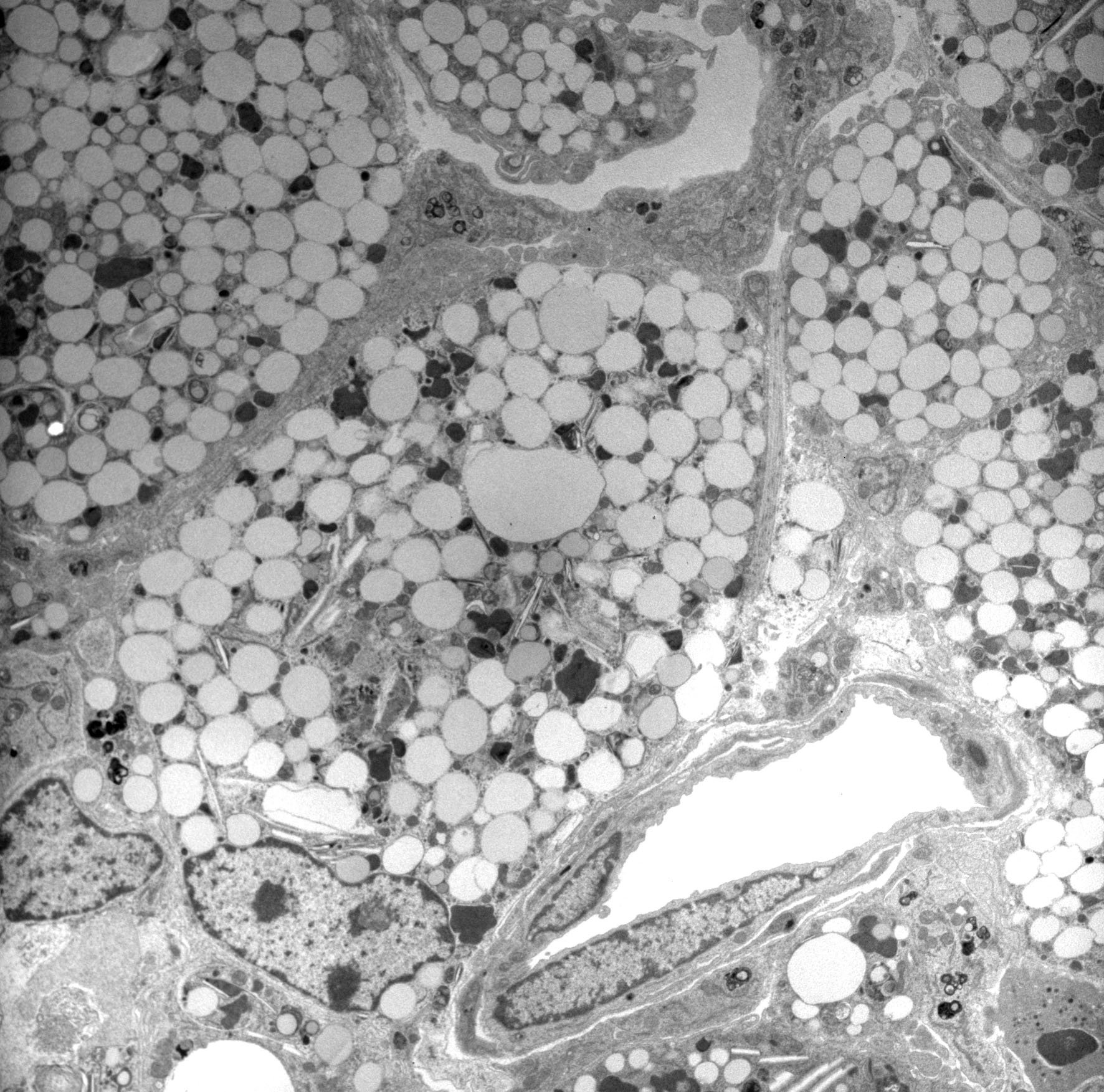
Currently, we are exploring the potential of targeting foam cells to enhance stroke recovery. Foam cells are a type of immune cell that forms in the brain after stroke in response to the breakdown of myelin, which can overwhelm the lipid processing capability of immune cells. In the weeks after stroke, foam cells contribute to the chronic inflammatory response that damages surrounding tissue and impairs recovery.
By selectively targeting foam cells, we aim to reduce chronic inflammation at the site of the stroke damage, promote tissue repair, and ultimately improve functional outcomes for stroke survivors. Our team is committed to developing innovative approaches to enhancing stroke recovery, and we are hopeful that this line of research will lead to new and effective therapies that can make a difference in the lives of stroke patients and their families.
T cell sitting on a foam cell
GFAP immunostaining
Dr. Kristian Doyle
Dr. Kristian Doyle
Dr. Kristian Doyle is an Associate Professor of Immunobiology and Neurology at the University of Arizona and a member of the Arizona Center on Aging.
He is an accomplished researcher with expertise in the fields of immunology, cardiovascular science, and neuroscience. He completed his postdoctoral training at Stanford University, where he investigated the role of the immune system in stroke and gained extensive experience in early-phase drug development.
Dr. Doyle has made significant contributions to the field of stroke research. He demonstrated that the inflammatory response to stroke lasts longer than the inflammatory response to equivalent sterile injuries to other parts of the body and that the processing of myelin lipid debris is a primary driving force. He has received several awards, including an American Federation of Aging Research Fellowship, an Anita Roberts Young Scientist Scholarship, and a K99/R00 faculty transition award from the National Institutes of Health. He is also a founding member of the Stroke-IMPaCT network, which is a team of researchers from universities across Europe and North America working together to identify novel therapeutic targets and improve outcomes for stroke patients.


